We, the Forbes lab, hosted a visiting scientist to the lab last week. We invited Dr. Arne Iserbyt (Fig.1) from Antwerp University in Belgium to collaborate on a project of my thesis. He showed me how to properly perform a particular protocol. The protocol that he taught me was to extract and Phenoloxidase and protein content from thoraxes of damselflies. The protocol was first developed by Dr. Robbie Stoks from the University of Leuven (Stoks et al. 2006). Arne used it during his thesis work (Iserbyt et al. 2012). Phenoloxidase concentration is one of the measures used to determine innate immune function in insects. It is an enzyme that activates melanogenesis that is the response to that the insect has to defends itself from certain parasites (Gonzalez-Santoyo & Cordoba-Aguilar 2011). Because I look at parasitism levels in damselflies, knowing the difference in levels of immune function between individuals and species is a good idea.
The protocol needs several steps: you must crush the thoraxes, add several buffers, centrifuge the mixture and place them onto a microplate adding more buffers and chemicals (Fig 2).
You also need a second microplate to determine appropriate levels of proteins. Then you insert into the microplate reader and wait patiently for absorption readings, the data that you will use (Fig 3).
While he was here, Arne also presented a seminar to the biology department about his thesis work.
He is an excellent collaborator and an excellent teacher. His instructions for the protocol that we tested and ran were very clear. It worked straight away and we had data after our first try. He was very encouraging allowing me to actually get my hands dirty (not literally) from the first test run.
Arne visited us for a week. This time was not all work. Since the lab work and protocols were working so well, we could have some time off to show the beauty of a Canadian fall. I decided to take Arne on a hike in Gatineau Park so that he could experience the wonderful fall colors. We were lucky because it was the perfect weekend for it, great weather and the leaves had not started to drop their leaves (Fig 4).
Hosting a visiting scientist is a great experience. It breaks the routine of regular lab work and actually injects new energy and enthusiasm because of sharing of ideas. I found that many new projects came to mind by having a different perspective come in. I learned a great deal from Arne. I hope Arne had a good time here as well.
References
González-Santoyo, I & Córdoba-Aguilar, A. 2011. Phenoloxidase: a key component of the insect immune system. Entomologia Experimentalis et Applicata 142: 1-16.
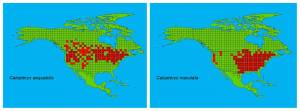
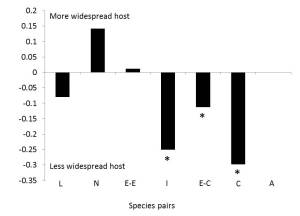
Iserbyt, A., Van Gossum, H. & Stoks, R. 2012. Biogeographical Survey Identifies Consistent Alternative Physiological Optima and a Minor Role for Environmental Drivers in Maintaining a Polymorphism. PLOS one 7: 1-10.
Stoks, R., De Block, M., Slos, S., Van Doorslaer, W., Rolff J. 2006. Time constraints mediate predator-induced plasticity in immune function, condition, and life history. Ecology 87: 809–815.